






What you discover when you examine the vaccine safety literature is that the safety of the vaccine program has not been established using sound, rigorous, independent science.
When public officials make the unqualified statement that vaccines are “safe and effective”, they are either misinformed or are not being openly transparent about the status of vaccine safety science.
Given that vaccines are a product given to healthy children, the level of safety testing ought to be even more rigorous than is required with all other pharmaceutical products. This is not the case. The safety testing of vaccine products is less rigorous, incomplete, and protocols appear to have been designed to obscure identifying long-term adverse effects of vaccines.
A product can be effective and not safe. Our history is replete with examples of this:
There is evidence that vaccines can be effective in reducing the incidence and symptoms of infections like measles and mumps, and thus I recognize the desire of government and public health to want to increase the use of vaccine products.
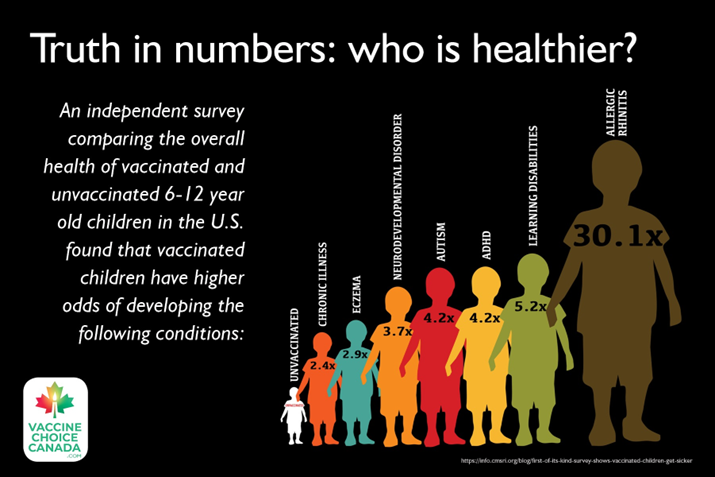
There is also evidence that vaccines can cause harm as they did to my son and to many other families. My concern is that governments and the medical industry have a tendency to over-simplify what is really a very complex matter. This over-simplification and allegiance to an ideology put us all at risk.
I wish to bring to your attention five issues pertaining to vaccine safety.

Most people, including doctors, nurses, politicians and public health officials, are not aware that vaccines are not tested for safety to the same standards required for all other medical products. Vaccines have been classified as ‘biologics’ and are exempted from the extensive safety testing required for all drugs.
The result is that no childhood vaccine product licensed for use in Canada has been tested for safety using the standards required of all other medical products. In other words, vaccines are not subjected to the long-term, double blind, placebo-controlled studies that are conducted on all other drugs prior to licensing. Instead, vaccines are released to the public with sub-standard safety testing.
Another concern is the unacceptably short time period for pre-licensing safety testing of vaccines. While pharmaceutical products are tested for safety for years prior to licensure, childhood vaccines undergo pre-licensing safety monitoring of a few days to a maximum of a few weeks.
This brief pre-licensing monitoring is not long enough to reveal whether vaccines cause autoimmune, neurological or developmental disorders like autism, learning disabilities, attention deficit hyperactivity disorder, life-threatening allergies, asthma, and other disorders. These disorders will only become apparent after the child is a few years of age.
Safety testing of childhood vaccines is conducted on a small sample, which may or may not include infants and children; is not compared against a control group receiving an inert placebo; and the period of testing ranges from as short as 48 hours to as long as 6 weeks. Here is an example of the duration of safety monitoring for various vaccine products licensed in Canada:3, 4
Hep B (Merck) – 5 days
Hep B (GSK) – 4 days
DTap – 8 days
MMR – 42 days
Polio – 3 days
Hib – 3 days
Pneumococcus – 7 days
Rotavirus – 8 days
Menningicoccal – 7 days Influenza – 4 days
COVID-19 – available before safety trials completed
Health Canada claims that it “conducts rigorous scientific review and testing of vaccines to assess their quality, safety, and efficacy before they are approved for use.” I contacted Dr. Teresa Tam, Canada’s Chief Medical Officer in 2018 to request evidence of the vaccine safety testing conducted by Health Canada. To date, Health Canada has failed to provide any evidence to support their claim of “rigorous scientific testing before they are approved for use.”
Our public health officials claim that the ‘artificial stimulation of the immune system’ with injected ingredients (vaccination) is “the safest, most effective and best way to protect our children and communities.” This opinion is not, however, supported by robust scientific evidence.
The fact is, we don’t know the safety of the current vaccination program because the science has not been done to the level that would support this conclusion. This is not my opinion, but rather the finding of the Institute of Medicine (IOM) which found that the safety of the current childhood vaccine schedule has never been proven in large, long-term clinical trials. They state:
In 1987, Congress mandated that Health and Human Services continuously improve the safety of vaccine products and report on their progress every two years. In 2018, in response to a Freedom of Information request, HHS admitted that it has failed to file even a single report over the 30 year period.
Even more disconcerting is that vaccine manufacturers have been granted legal immunity for any harm or deaths caused by their products.
Think about this for a moment. Vaccines are the only product, medical or otherwise, where a manufacturer is not legally responsible for injury and death caused by their products. The result of this legal immunity is that no one is held accountable when injuries and deaths occur. Would you accept this lack of accountability with any other product? Why do we permit this lack of accountability with something as important as childhood vaccines? And finally, if vaccines are as safe as claimed, why do vaccine manufacturers need immunity?
A consequence of this legal immunity is that there is no legal or financial incentive for the medical industry to make their products safer, even when there is clear evidence that vaccines can be made safer.
“We and our children have been and are the victims of a
carefully orchestrated, programmed propaganda campaign
in which maximum publicity is repeatedly given
to rare complications from one of the childhood diseases
while actively suppressing
the cases of morbidity and death caused by vaccines.”
~ Dr. Thomas Stone, MD